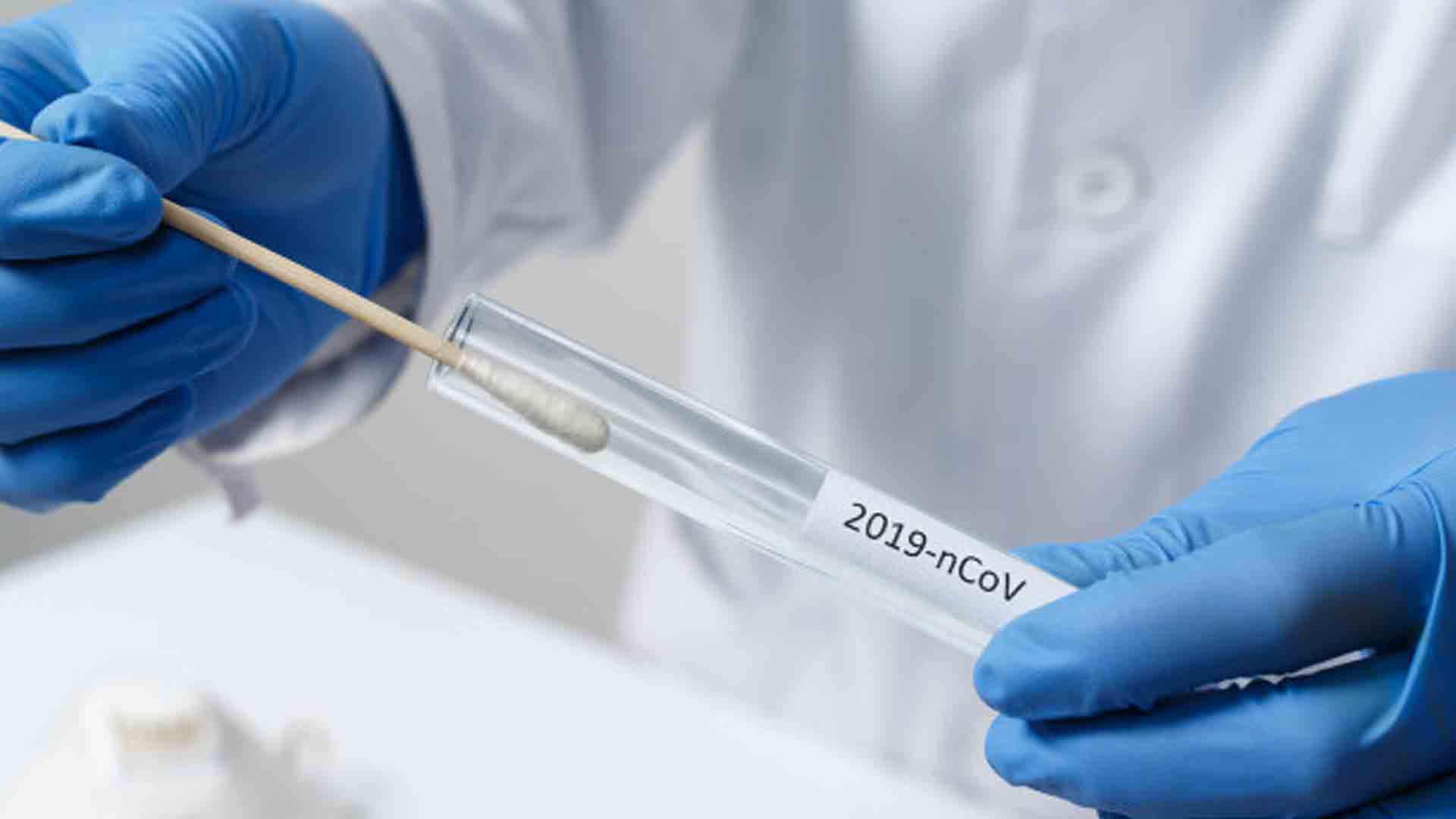
The use of self-administered Covid-19 test kits does not require healthcare workers to perform nasopharyngeal or oropharyngeal swab collection.
In a televised Laging Handa public briefing, Research Institute for Tropical Medicine (RITM) Laboratory Chief Armando Tandoc III said the test kits will require nasal samples only.
“Kapag po nasal, galing lamang po ito sa harapan o kaya hanggang gitnang bahagi ng ilong na maaaring kunin sa sarili o kung sa may kapansanan o sa mga bata ay maaaring kunin ng isang nakakatanda (When you say nasal, the sample is from the front or up to middle part of the nose of an individual, in case of children or persons with disabilities any adult can assist them),” Tandoc said.
He added that some test kits will require saliva samples.
“So, sa pagsagawa naman po ng test mismo, may mga instruction naman na susundin kung paanong ilagay iyong sample sa cartridge at kung gaano katagal iyong hihintayin bago magkaroon ng resulta (in performing the test, there are instructions on how to place the sample in the cartridge and how long you need to wait for the result),” he said.
Earlier, the Department of Health said it will release guidelines on the proper use of the self-administered or home test kits.
On Monday, the Food Drug Administration (FDA) said it approved two kits — the “Panbio Covid-19 Antigen Self-Test” of Abbott which will be available in boxes of 1, 4, 10, and 20 tests and “SARS-CoV-2 Antigen Rapid Test” of Labnovation which may be bought in boxes of 2, 5, and 20 tests.
Apart from the two approved kits, the FDA endorsed 31 more kits to the RITM.
Tandoc emphasized that users of the kits “must read and follow carefully” the instructions that come with them.
“Kapag po kasi hindi nasunod ito mula sa pagkolekta hanggang sa paggawa ng test at pag-interpret dito, maaring makaapekto po sa accuracy (If the instructions are not followed starting from sample collection up to testing and interpretation, the accuracy might be affected),” he said. (PNA)